.png)
Welcome to Affy
GMP ISO 9001:2005 Certified Co.
Pharmaceutical & Nutraceutical Manufacturing Experts.
Why Choose Affy ?
25+ Years of Pharmaceutical & Nutraceutical Manufacturing Excellence Backed by decades of expertise, advanced facilities, and strict quality standards to meet global healthcare needs.
Quality Focus
Quality Assurance & Compliance Strict GMP guidelines and quality control processes to ensure safe and effective pharmaceutical products.
Manufacturing Strength
Advanced Manufacturing Facilities State-of-the-art infrastructure supporting efficient, scalable, and compliant pharmaceutical production.
GMP & ISO Certified Operations
GMP & ISO Certified Operations Manufacturing processes aligned with national and international quality standards.
About Us
Affy Group is a leading Indian multinational pharmaceutical company headquartered in Baddi, Himachal Pradesh, India. With over 25 years of manufacturing excellence, the Group has established itself as a trusted name in the pharmaceutical industry.
Affy Group of Companies records an annual turnover of approximately INR 1 Billion, driven by a highly skilled and committed workforce of over 650 professionals. Our operations are supported by cGMP- and ISO 9001:2015–certified facilities and span multiple integrated divisions.
We serve a strong and diverse customer base of nearly 800 clients, offering a portfolio of more than 6,000 brands across domestic and international markets. Affy operates three state-of-the-art manufacturing units comprising 13 specialized production sections, further supported by two dedicated packaging and printing units.
Affy specializes in the manufacturing and marketing of pharmaceutical products across key therapeutic segments, including Pain Management, Anti-Infectives, Paediatrics, Gynaecology, Gastrointestinal, and Beta-Lactam formulations.
Our dedicated, dosage-form–specific manufacturing facilities enable us to meet high production demands while consistently delivering quality, safety, and regulatory compliance. At Affy, innovation, quality, and patient well-being remain at the core of everything we do.
We maintain large-scale annual production capacities, including 2.4 billion tablets (General, Beta-Lactam and Non-Beta-Lactam), 1 billion soft gelatin capsules, 18 million dry syrups (Beta-Lactam and Non-Beta-Lactam), and 1.2 million injectable units, supported by advanced infrastructure and dedicated manufacturing lines.
Our Facilities
Explore our world-class manufacturing units and corporate office that together power innovation, quality, and trust in pharmaceutical manufacturing.

Affy Parenterals – Manufacturing Unit
Affy Parenterals, established in Baddi, maintains a compliant and well-equipped manufacturing facility for large-scale tablet, capsule, and injectable production.
Affy Pharma Pvt Ltd
Affy Pharma, established in Baddi, maintains a compliant and well-equipped manufacturing facility.
Medilinks
Medilinks, established in Baddi, maintains a compliant and well-equipped manufacturing facility.
ECS Technologies
ECS Technologies, established in Baddi, maintains a compliant and well-equipped manufacturing facility.
Avans Healthways
Avans Healthways, established in Baddi, maintains a compliant and well-equipped manufacturing facility.
Corporate Office
Based in Noida, the Corporate Office serves as the central hub for corporate governance, regulatory affairs, quality oversight, and business operations.
Brand Manufactured
Sections
Production Unit
Certifications & Approvals
Products
Explore our wide range of pharmaceutical product categories designed to meet evolving healthcare needs.

Injection
High-quality injectable pharmaceutical products manufactured under strict GMP and ISO standards, ensuring sterility and safety.
More Info
Tablets
Our tablet manufacturing unit is equipped with modern machinery and follows strict GMP and ISO standards, delivering tablets with precise dosing, uniformity, and assured quality.
More Info
Capsules
Our capsule manufacturing facilities are equipped with advanced technology and follow strict GMP and ISO standards to deliver safe, reliable, and high-quality capsule formulations.
More Info
Syrup
Syrup Manufacturing (Beta-Lactam / Non-Beta-Lactam) – 18 Million Units GMP-compliant manufacturing of Beta-Lactam and Non-Beta-Lactam dry syrups with an annual capacity of 18 million units, ensuring consistent quality, safety, and dosing accuracy.
More Info
Beta lactum tablet
Our Beta-Lactam tablet manufacturing facility is designed to meet the highest standards of quality, safety, and regulatory compliance. We operate in fully segregated Beta-Lactam production areas to prevent cross-contamination and ensure patient safety.
More Info
General Tablet
The facility is designed with controlled environments, validated equipment, and efficient workflows to support large-scale and consistent tablet production. Advanced granulation, blending, compression, and coating systems are used to achieve uniformity and precision in every batch.
More InfoContract Manufacturing
Partner with us for end-to-end contract manufacturing solutions, from formulation development to final packaging, meeting national and international regulatory standards. We support our partners with scalable manufacturing capacity, strict quality assurance, and transparent processes to help bring safe, effective pharmaceutical products to market efficiently.
Tablet Manufacturing Excellence
We specialize in high-quality pharmaceutical tablet manufacturing with a strong focus on precision, consistency, and regulatory compliance. Our processes are designed to deliver reliable products that meet industry standards and client expectations.
Quality-Controlled Tablet Production Our tablet manufacturing operations follow strict GMP guidelines, supported by validated processes, in-process quality checks, and comprehensive final testing. This ensures uniformity, stability, and safety across every batch produced.
Trusted Manufacturing Excellence
We are committed to delivering reliable pharmaceutical manufacturing solutions with a strong focus on quality, compliance, and consistency. Our processes are designed to meet industry standards while supporting our partners with dependable and transparent operations.
Quality-Driven Operations We ensure strict adherence to GMP guidelines through controlled manufacturing environments, validated processes, and continuous quality monitoring. Our experienced team works diligently to maintain product integrity, safety, and regulatory compliance at every stage of production.
Nutraceutical Manufacturing Excellence
We specialize in high-quality nutraceutical manufacturing with a strong focus on safety, efficacy, and regulatory compliance. Our processes are designed to deliver reliable nutraceutical products that meet industry standards and consumer expectations.
Quality-Controlled Nutraceutical Production Our nutraceutical manufacturing operations follow stringent quality guidelines, supported by controlled environments, validated processes, and thorough quality checks. This ensures consistency, stability, and safety across every batch produced.

Injection Manufacturing Excellence
We specialize in pharmaceutical injection manufacturing with a strong focus on sterility, safety, and regulatory compliance. Our processes are designed to deliver high-quality injectable products that meet stringent industry and patient safety standards.
Our injection manufacturing operations follow strict GMP guidelines, supported by aseptic processing, controlled cleanroom environments, and comprehensive quality testing. This ensures product sterility, stability, and consistency across every batch produced.
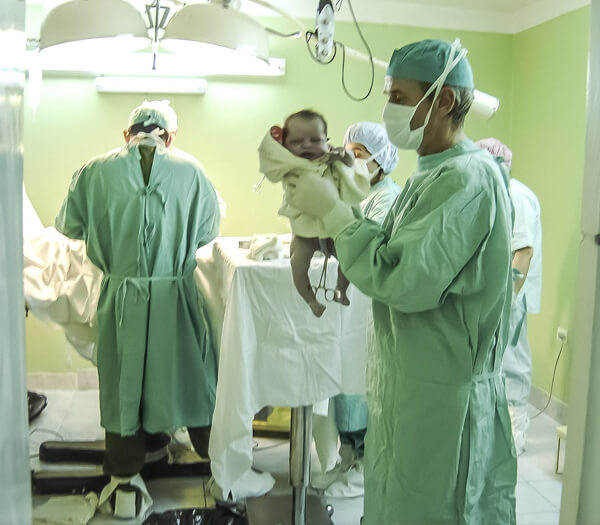
Ointment Manufacturing Excellence
We specialize in high-quality pharmaceutical ointment manufacturing with a strong focus on safety, consistency, and regulatory compliance. Our processes are designed to deliver effective topical formulations that meet industry standards and patient needs.
Our ointment manufacturing operations follow strict GMP guidelines, supported by controlled processing conditions, validated mixing and filling processes, and comprehensive quality checks. This ensures uniformity, stability, and safety across every batch produced.

Marketing Division
We operate through 4 specialized marketing divisions across multiple therapeutic segments.




Manufacturing Insights
Comprehensive information about our pharmaceutical and nutraceutical manufacturing operations, quality systems, and compliance standards.
Are your manufacturing facilities WHO-GMP certified?
Yes, our manufacturing plant operates in compliance with WHO-GMP guidelines. We follow stringent quality systems, validated processes, and regulatory standards to ensure safe, effective, and consistent products.
What quality control measures are followed during production?
Our facility is equipped with in-house Quality Control (QC) laboratories. Raw materials, in-process samples, and finished products undergo rigorous testing to meet pharmacopeial and regulatory requirements.
What types of dosage forms do you manufacture?
We specialize in manufacturing a wide range of dosage forms, including: Tablets & Capsules Liquid Orals (Syrups & Suspensions) Injections Nutraceutical products All products are manufactured under controlled environments.
How do you ensure hygiene and contamination control in the plant?
Our plant follows strict hygiene protocols, including: Controlled cleanroom environments HEPA filtration systems Personnel gowning procedures Regular sanitation and validation processes These measures help maintain product integrity and patient safety.
Do you support third-party or contract manufacturing?
Yes, we offer third-party and contract manufacturing services. We work closely with clients to meet customized formulations, packaging, and regulatory requirements.
How is regulatory compliance and documentation managed?
We maintain complete batch manufacturing records, validation documents, SOPs, and stability data. Our regulatory team ensures compliance with applicable national and international guidelines.
Our Customers
Some of Our Esteemed Customers Who Trust Our Quality and Commitment
Gallery
Take a look inside our modern pharmaceutical manufacturing and quality facilities.
Contact
Get in touch with Affy Pharma for product inquiries, contract manufacturing, or partnership opportunities. Our team is ready to assist you with reliable and compliant pharmaceutical solutions.
Location
H-206, H Block, Sector 63, Noida, Uttar Pradesh 201301
Call Us
+91 9511351135
Email Us
info@affygroup.com